
Drugs are chemical compounds used to treat many illnesses. But it can worsen the situation if it is contaminated. If a drug gets contaminated by microbes, it can cause immediate and long-term harm to patients treated with that drug. Microorganisms can also alter the chemistry and pharmacology of drugs, which breaks down the active ingredients and impacts the product’s efficacy.
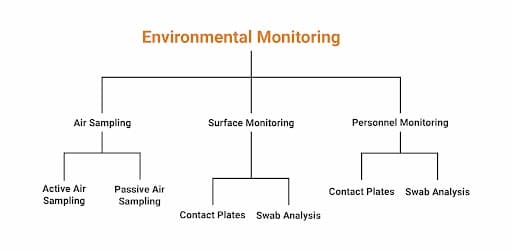
In a manufacturing facility, key sources of contamination are the air, equipment, surfaces, or even the person performing the activity. And to avoid these contaminations, the manufacturing facility should regularly monitor the air, equipment, surfaces, or personnel wearables.
So now the question is:
How can an industry ensure the quality of the environment in the manufacturing facility?
Here comes aseptic processing.
Aseptic Processing
Every pharmaceutical industry follows aseptic processing. Aseptic processing assists the pharmaceutical industry in producing contamination-free pharmaceutical products.
Pharmaceutical companies follow guidelines from organizations like the FDA and the European Medicines Agency (EMA) to implement an Environmental Monitoring program to ensure that aseptic processing is possible.
Environmental monitoring
Microbiological Environmental Monitoring is a method of demonstrating the microbiological quality of a controlled environment and detecting changes in a timely manner. It involves gathering data on the basis of the number of microbes recovered from air, surfaces, and humans. Such samples are collected from the cleanroom area and exit airlock.
Microbiological testing, when combined with strict environmental monitoring, ensures that pharmaceutical sterile products such as injections, infusions, and ophthalmic solutions, as well as non-sterile products such as solutions, capsules, tablets, and ointments, are safe and effective. Environmental monitoring is required for the GMP process and is part of the regulatory compliance process. It must be provided in order for the final product to be released.
According to the Pharmacopoeia guidelines, all Environmental Monitoring programs should employ a combination of the following methods:
Microorganisms in the air increase the risk of cross-contamination of ingredients and products; that’s why the air quality of any lab or production area is important to monitor. According to the CDC, biological contaminants in the air can include particles, bacteria, fungi, viruses, and pollen.
For non-viable particles, air is sampled by laser particle counters, whereas for viable particles, Active Air sampling as well as Passive Air Sampling methods are followed.
In this method, an Air Sampler is used to collect an established volume of cleanroom air on a petri dish containing culture medium. Once the sampling is done, the plates are then incubated for the required time and temperature.
This method involves exposing the settle plates to air for a certain period of time, and just like active air sampling, after sampling completes, the plates are incubated for the required time and temperature.
No matter how thoroughly a lab or cleanroom has been sanitized, microorganisms can quickly transfer from one surface to another via air, touching the surface with something contaminated.
Surface Monitoring is performed by Contact Plates and Swab Analysis.
Contact Plates
Ready-to-Use Contact Plates, or RODAC Plates are used to collect samples from the surface area of around 25 cm2. The sample is taken by gently rolling the raised surface of the agar plate onto a flat surface for a defined time. After sampling, the plates are incubated for the required time and temperature.
Swab Analysis
This method is used to obtain samples from an irregular surface. Where the analyst takes samples using a template made up of SS with a measured area of 25 cm2 for sampling. After sampling, the swab sample is then transferred to the respective growth media plates and incubated for the required time.
Humans are the biggest source of contaminants in an aseptic environment, even with good hygiene habits and the right PPE kits.
Personnel Monitoring is done in a highly controlled area where the surrounding environment is of class B and the working environment is of class A.
The person who is entering the classified section should be qualified for gowning. He or she should know and follow the gowning procedure. It is necessary because each individual can bring particulate and microbial contaminants into the area.
According to the regulations, the personnel should be monitored after critical operations in the exit airlock.
Similarly, like Surface Monitoring, Personnel Monitoring is also performed using Contact Plates and Swab Analysis.
Contact Plates
Ready-to-Use Contact plates or RODAC Plates are used to collect samples from the person. The sample is collected by gently rolling the raised surface of the agar plate onto the gown. On the gown the samples are collected from the locations that are most prone to contamination. After sampling, the plates are incubated for the required time and temperature.
Swab Analysis
Like Contact plates, in this method, the samples are collected from the locations on the gown that are most susceptible to contamination. After sampling, the swab is then transferred to a medium containing growth medium and incubated for the required time.
The growth of the organisms in the form of colony-forming units is then observed in these all-incubated plates.
The Limits
There are standard limits for microbial growth observed in each grade of clean room environment.
Fulfilling Environmental Monitoring Needs
TM Media offers quality Environmental Monitoring solutions to ensure that your work meets compliance and regulatory standards and that you feel confident about the products you’re releasing on the market.
Our “Absolute EM” category is specifically designed for Environmental Monitoring; it includes the Microbial Air Monitoring System (TME 003, TME 004, TME 005) and Sterile Gamma-irradiated Ready-To- Use plates of 90 mm and 55 mm.
We also provide a wide range of culture media and other products specifically designed for various pharmaceutical testing processes. All the products are manufactured in accordance with USP/EP/JP/IP.
Along with Environmental Monitoring we also provide products tailored for the pharmaceutical industry for various other activities, such as Combo Sterility Kit (TMCK 001) for sterility testing, Soybean Casein Digest Medium (TMH 102) for media fill activities, and many more.

The specter of antimicrobial resistance (AMR) hangs over modern medicine. Antibiotics – once a miracle are losing their power as...
Read More
We are thrilled to announce that TM Media has been recognized as the LeadingBiotechnology Company at the prestigious 6th Elets...
Read More
Think of a field as a big garden where tiny superheroes, called microbes, work behind the scenes. They’re not just...
Read More
Maintaining the highest standards of quality and safety is paramount in pharmaceutical manufacturing. Strict adherence to current good manufacturing practices...
Read More
Potato Dextrose Agar (PDA) is a widely utilized medium in microbiology, specifically designed for the isolation and enumeration of yeasts...
Read More
In the complicated world of microbiology, where precision and reliability are of utmost importance, Soybean Casein Digest Agar (SCDA) stands...
Read More